PharmaPMS
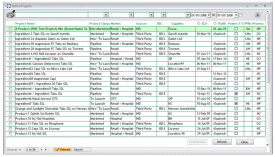
A business information tool for monitoring generic pharmaceutical projects during the pre and post launch period, bringing together information from areas such as: Artwork, Portfolio, Patent, Planning, Product Management, Quality, Regulatory and Sourcing. Users are assigned “permissions” allowing full access, read only or no access to individual pages, restricting the data to those that should have access to it. A unique feature is the “Actions and responses” system - designed to let the user know of any outstanding actions they have been set. These actions are particular to a Project and assigned to a user, allowing a start up view of all outstanding actions. Many standard reports can be produced to give printed output, including launch charts, Patent reports and Launch Date changes.
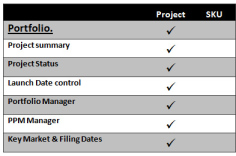
Portfolio Management
A key feature within PharmaPMS, allowing the pharmaceutical portfolio professional to manage large numbers of projects with critical dates and key contacts in addition to a project summary. This key information is then repeated to other pages allowing others to view but not edit, hence sharing but protecting important information
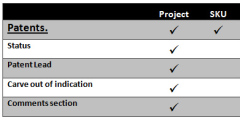
Patent Management
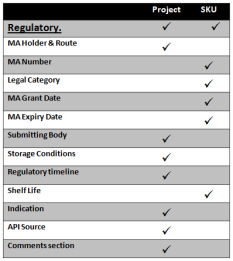
Regulatory Affairs
Details of Regulatory information including details of indications, a summary of MA Numbers & Grant Date, Storage conditions, Regulatory Time lines, MA Holder, MA Route, Submitting Body, Carve out dates and approval & selection of relevant sites. Items such as selection of API sites and Indications are repeated to other pages such as Patent allowing full interactivity with other departments. A Regulatory Comments section caters for notes specific to regulatory dealings on the project .
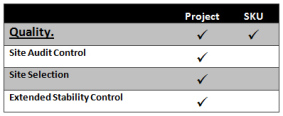
Quality
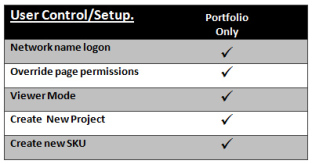
User Control
Consideration is given to controlling access to certain users. Each user is allocated a “User Type” and is associated with the Network logon name. This can hide or make “Read only” pages of information. A viewer mode (cost free user) with a limited display is also configurable. New Projects and SKU’s can be created with no practical limit to numbers of projects or SKU’s.